AFm is abbreviated from ‘alumina, ferric oxide, monsulfate’. The AFm phase of Portland cement refers to a group of similar members of hydrated calcium aluminates that have a hydrocalumite-like structure based on 4CaO.Al2O3.13–19 H2O.

Image Credit: Natee Photo/Shutterstock.com
As a result, many hydrated types of cement contain AFm phase combinations. AFm phases have been synthesized from precursors, and experimentally determined phase relationships are shown at 25°C. AFm refers to a family of hydrated calcium aluminate phases, which are structurally similar to hydrocalumite.
These phases are known to have variable hydration states with altering water content depending on the relative humidity (RH), anion type, and temperature, which may be linked to the volume variations causing shrinkage and swelling.
There are numerous hydration states, and the most significant include: monosulfoaluminate, hydroxy AFm, stratlingite, hemicarboaluminate, and monocarboaluminate. The thermodynamic features are related to changes in their water content during absorption/desorption. Unfortunately, the stability conditions of these phases are not well documented or researched.
Understanding the Structure of AFm Phases
AFm is one of the principal products that is created during the process of hydration of Portland and calcium aluminate cement-based systems. The formula for AFm is represented by 4CaO.Al2O3.13–19 X H2O, with X equating to an exchangeable singly charged chloride or half of a doubly charged anion carbonate and sulfate.
In this instance, sulfate and carbonates can be substituted for hydroxide (OH-). From a mineralogical standpoint, they behave as separate phases and these compositions do not form solid solutions, except a limited replacement (50 mol%) of sulfate by hydroxide. As a result, many hydrated types of cement will have an AFm phase combination.
A paper by Luis Baquerizo et al. has discovered new experimental findings on the hydration states of the most important AFm phases. Additionally, the findings on thermodynamic properties associated with the desorption and absorption that change the water content are also discussed.
The Most Significant AFm Phases
Monosulfoaluminate (Ms)
Monosulfoaluminate (Ca4Al2(SO4)(OH)12∙6H2O) has a vital role in the binding of Portland cement by interchanging the original interlayer ions 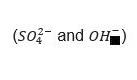 with chloride ions. Commonly, monosulfoaluminate is a cement hydrate that is created by the reaction that first forms Ettringite.
with chloride ions. Commonly, monosulfoaluminate is a cement hydrate that is created by the reaction that first forms Ettringite.
The reaction with residual tricalcium aluminate during the hydration of cement occurs in blocks of cement with a calcium carbonate content of 1%. Its molecular structure closely resembles that of kuzelite (C4AŜH12), a naturally occurring mineral with a crystal-like structure.
Hydroxy-AFM (OH-AFm)
Hydroxy-AFM has a formula of C4AH7+x, where x is the interlayer water content and can vary from anywhere between 0 and 12, with two anion (OH) groups. With respect to the mixtures of portlandite and hydrogarnet, hydroxy AFm at all temperatures is thermodynamically metastable. However, there are discrepancies in terms of water content and corresponding layer thickness at different conditions.
The presence of carbonates and sulfates makes the occurrence of hydroxy-AFm in hydrated Portland cement unlikely, resulting in the precipitation of monosulfoaluminate and carboaluminate phases.
Strätlingite (Str)
Gehlenite hydrate is the common name for Strätlingite (C2AŜH8). It occurs as a hydration product in fly ash blended blocks of cement, metakaolin- or slay, and also in hydrated high alumina cement. AFm phases have an identical principal layer constitution to Strätlingite, which has a chemical formula of C2AŜH7.25 and an aluminosilicate anion as an interlayer, which consists of a double tetrahedral
However, our knowledge and understanding of its compatibility and stability with other phases are limited. Strätlingite maintains its stability in aluminosilicate-rich cement hydrate systems. Its stability usually declines with increasing temperatures, having an upper limit of stability of approximately 95°C. Strätlingite progressively decomposes into various solids, mostly hydrogarnet solid solutions above this temperature.
More from AZoBuild: Analyzing the Water-Cement Ratio of Concrete with Electron Microscopy
Hemicarboaluminate (Hc)
Hemicarboaluminate is formed as one of the key phases during the beginning of the cement reaction process. Under room temperature and typical experimental conditions, two significant cementitious phases are detected, namely hemicarboaluminate (HC), which is a major phase, and carbonated calcium hemicarboaluminate (cHc). As the temperature rises, the Hc phase changes into cHc.
Due to the slight differences in interlayer water or limited solid solutions, Hc exhibits slight differences in basal spacing compared to other AFm phases.
Conclusion and Future for AFm Research
There are no full experimental data or thermodynamic characteristics for changes in hydration states in AFm phases at this moment. More research is needed to obtain data that will help assess the stability of complex cementitious systems containing AFm phases under unsaturated conditions.
Furthermore, the data will allow for the prediction of the mineralogical composition of cement pastes derived from novel binder combinations, as well as the prediction of phase shifts in concrete buildings as a result of environmental changes (for example severe drying at extreme temperatures, etc.).
This information will allow engineers to model the reaction of cementitious systems during drying and wetting, and therefore design systems that are more resistant to extreme environmental conditions.
References and Further Reading
Baquerizo, LG, Matschei, T, Scrivener, KL, Saeidpour, M & Wadsö, L 2015, 'Hydration states of AFm cement phases', Cement and Concrete Research, vol. 73, pp. 143-157. https://www.sciencedirect.com/science/article/pii/S0008884615000538?via%3Dihub
Matschei, T, Lothenbach, B & Glasser, FP 2007, 'The AFm phase in Portland cement', Cement and Concrete Research, vol. 37, no. 2, pp. 118-130. https://www.sciencedirect.com/science/article/pii/S0008884606002705?via%3Dihub
Runčevski, T., Dinnebier, R.E., Magdysyuk, O.V. and Pöllmann, H., 2012. Crystal structures of calcium hemicarboaluminate and carbonated calcium hemicarboaluminate from synchrotron powder diffraction data. Acta Crystallographica Section B: Structural Science, 68(5), pp.493-500.
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.