Feb 2 2009
Your refrigerator's humming, electricity-guzzling cooling system could soon be a lot smaller, quieter and more economical thanks to an exotic metal alloy discovered by an international collaboration working at the National Institute of Standards and Technology (NIST)’s Center for Neutron Research (NCNR).
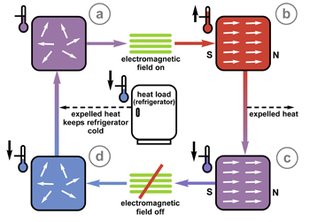 A magnetocaloric material [bottom] heats up when magnetized (b); if cooled and then demagnetized (c), its temperature drops dramatically (d). NIST scientists may have found a way to use magnetocalorics in your fridge. Credit: Talbott, NIST
A magnetocaloric material [bottom] heats up when magnetized (b); if cooled and then demagnetized (c), its temperature drops dramatically (d). NIST scientists may have found a way to use magnetocalorics in your fridge. Credit: Talbott, NIST
The alloy may prove to be a long-sought material that will permit magnetic cooling instead of the gas-compression systems used for home refrigeration and air conditioning. The magnetic cooling technique, though used for decades in science and industry, has yet to find application in the home because of technical and environmental hurdles—but the NIST collaboration may have overcome them.
Magnetic cooling relies on materials called magnetocalorics, which heat up when exposed to a powerful magnetic field. After they cool off by radiating this heat away, the magnetic field is removed, and their temperature drops again, this time dramatically. The effect can be used in a classic refrigeration cycle, and scientists have attained temperatures of nearly absolute zero this way. Two factors have kept magnetic cooling out of the consumer market: most magnetocalorics that function at close to room temperature require both the prohibitively expensive rare metal gadolinium and arsenic, a deadly toxin.
But conventional gas-compression refrigerators have their own drawbacks. They commonly use hydrofluorocarbons (HFCs), greenhouse gases that can contribute to climate change if they escape into the atmosphere. In addition, it is becoming increasingly difficult to improve traditional refrigeration. “The efficiency of the gas cycle has pretty much maxed out,” said Jeff Lynn of NCNR. “The idea is to replace that cycle with something else.”
The alloy the team has found—a mixture of manganese, iron, phosphorus and germanium—is not merely the first near-room-temperature magnetocaloric to contain neither gadolinium nor arsenic—rendering it both safer and cheaper—but also it has such strong magnetocaloric properties that a system based on it could rival gas compression in efficiency.
Working alongside (and inspired by) visiting scientists from the Beijing University of Technology, the team used NIST’s neutron diffraction equipment to analyze the novel alloy. They found that when exposed to a magnetic field, the newfound material’s crystal structure completely changes, which explains its exceptional performance.
“Understanding how to fine-tune this change in crystal structure may allow us to get our alloy’s efficiency even higher,” says NIST crystallographer Qing Huang. “We are still playing with the composition, and if we can get it to magnetize uniformly, we may be able to further improve the efficiency.”
Members of the collaboration include scientists from NIST, Beijing University of Technology, Princeton University and McGill University. Funding for the project was provided by NIST.
* D. Liu, M. Yue, J. Zhang, T.M. McQueen, J.W. Lynn, X. Wang, Y. Chen, J. Li, R.J. Cava, X. Liu, Z. Altounian and Q. Huang. Origin and tuning of the magnetocaloric effect for the magnetic refrigerant MnFe(P1-xGex). Physical Review B. Vol. 79, 014435 (2009).